Disease Manifestation – Proteins to Cells
Neurofibromatosis type I mainly impacts one protein, neurofibromin. Through this protein, however, NF1 is able to exert a multitude of symptoms, such as tumor growth, along the whole body of someone with the disease. This powerful ability is due to the regulatory importance of neurofibromin in specific nervous-system-associated cells around the body. NF1 leads to the development of neurofibromas around the body. By finding the specific types of cells that comprise neurofibromas, insight can be gained into the types of cells important for the proliferation of NF1, elucidating mechanisms responsible for the pathogenesis of the disease. Furthermore, performing genetic and proteomic analyses on these cells will help determine how NF1 is able to hijack bodily cells for the progression of disease.
Neurofibromas are comprised mostly of cells expressing S100 protein (S100+ cells), which tend to be Schwann cells.1 Schwann cells are non-neuronal cells in the nervous system, crucial for maintaining and regulating neuronal cells. By isolating S100+ cells from neurofibromas and performing a genetic analysis, it was found that the NF1 gene was frequently mutated and inactivated in the isolated cells.1 This result indicates that a defect in the NF1 gene affects S100+ cells in neurofibromas, and that they are primarily responsible for the pathogenesis of the benign tumors.1 In addition to defective S100+ cells, neurofibromas were also found to contain abnormal S100– cells, suggesting further mechanisms of disease progression that go beyond the inactivation of the NF1 gene product (although these mechanisms are likely not primarily responsible for the pathogenesis of the disease).1 By disrupting the function of Schwann cells through the production of ineffective neurofibromin, the crucial role of Schwann cells in providing support to the peripheral nervous system is compromised. The lack of myelinating Schwann cells may lead to deterioration of nervous system function, the inability to control neuronal cell growth, and the lack of key quality-control checkpoints for nervous system cells.2
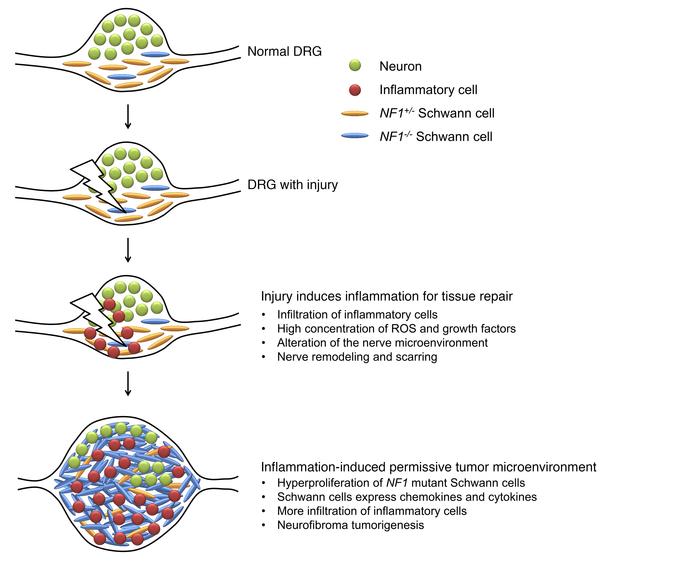
Mast cells and macrophages were also found to be crucial for the growth and survival of plexiform neurofibromas.3 The inflammatory nature of neurofibromas provides insight into the reasons behind this relationship. Plexiform tumor regression was found to occur as a result of a reduced number of macrophages in the neurofibromas, suggesting the crucial role of pro-inflammatory macrophages in tumor growth.3 Mast cells were found to be crucial for remodeling the microenvironment of neurofibromas through the upregulation of a key signaling pathway, enabling tumor growth.3
The growth of tumors is indicative of unregulated cell growth, explaining much about how NF1 affects the body so significantly. Through genetic mutations rendering neurofibromin ineffective, NF1 is able to progress so rapidly through breaking key cell-signaling pathways that allow for proper regulation, coordination, communication, and function of cells. Through mutations in the GAP neurofibromin, NF1 is able to affect and misregulate a multitude of cellular pathways, most of which are cytosolic. Most of the pathways affected by NF1 are crucial for the recruitment of immune cells to a site of infliction, or in the proliferation and differentiation of certain cell types.
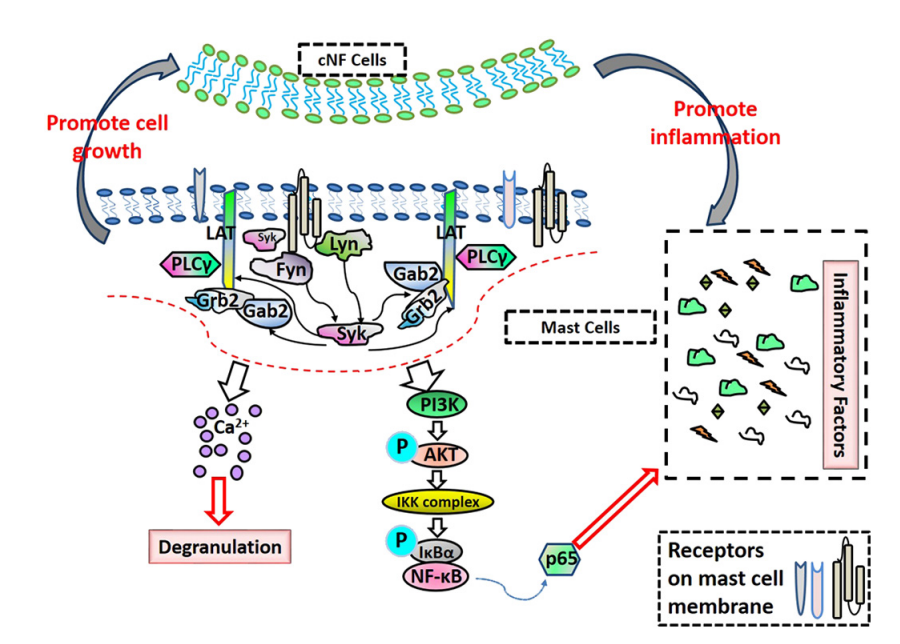
Neurofibromas were found to activate mast cells and increase inflammation through upregulation of the PLCγ/AKT/IκBɑ/p65 signaling pathway in mast cells.4 Mast cells with a loss-of-function mutation of the NF1 gene exhibited elevated levels of histamine, IL-6, LTC4, and TNF-𝛼, which are inflammatory mediators generated during mast cell activation.4 Elevated levels of these inflammatory mediators were found to promote the levels of p-PLCγ, leading to upregulation of the PLCγ/AKT/IκBɑ/p65 pathway and therefore, the mast cell inflammatory response.4 These upregulated mast cells promote the growth of neurofibromin-deficient S100+ cells through activation of the PLCγ/AKT/IκBɑ/p65 pathway, enabling tumor growth.4
Neurofibromin deficiency is also linked to an upregulation of the Ras/ERK signaling pathway in malignant peripheral sheet tumors.5 Malignant peripheral sheet tumors (MPNSTs) are what plexiform neurofibromas can eventually progress into (the progression from benign to malignant tumor, however, isnot very common in NF1). Depletion of neurofibromin in cells was found to increase the levels of phosphorylated extracellular regulatory kinase (ERK) 1 and 2.5 Neurofibromin deficiency is able to increase the levels of phosphorylated ERK through modulation of SP1, a key transcription factor of the EGFR gene (extracellular growth factor).5 Phosphorylated SP1 was found to translocate to the nucleus, acting as a transcription factor and promoting EGFR expression.5 A deficiency in the GTPase, neurofibromin, does not allow for the dephosphorylation (and therefore, the inactivation) of SP1, leading to significant overexpression of EGFR.5 Upregulation of EGFR through a deficiency in neurofibromin is crucial to the progression of plexiform neurofibromas into MPNSTs.5 Generally, neurofibromin inhibits EGFR growth by inhibiting Ras in the stated pathway. STAT3 signaling is strongly associated with creating inflammatory environments that allow for tumors to flourish and proliferate in.3 STAT3 was found to regulate essential cytokine-chemokine signaling in neurofibromas, required for their survival.3 Furthermore, the JAK/STAT3 pathway is upregulated by EGFR, which will further contribute to the progression of benign neurofibromas into malignant MPNSTs.
The cells and pathways affected by NF1 provide insight into why this disease is so powerful, yet the mutations are sequestered to such few places. The presence of Schwann cells around the body, and the dependency of cells on the described signaling pathways make it clear that without neurofibromin, a cascade of deleterious effects should be expected. Although the biochemical nature of NF1 is understood better, the variability in onset of disease, as well as the difficulty of diagnosing prior to physical onset makes prevention nearly impossible, and intervention and treatment extremely difficult.
Works Cited
For a complete list of all references used, please access the Annotated Bibliography
6 Comments
Alyssa Horwitz · May 8, 2022 at 5:57 am
Michael – I appreciated exploring your pages and learning how the widely-studied cancer-associated Ras-MEK-ERK pathway is regulated in NF1. While the most evident clinical presentation is dermatological, it sounds like there are other manifestations of NF1 such as cognitive and behavioral impairments. Can the biochemical processes governing these outcomes also be explained through Ras-dependent signaling? Are there any examples of neurofibromin regulation that are Ras-independent?
Miranda Robinson · May 8, 2022 at 3:54 pm
Hey Mike! This is all really fascinating. I find it interesting that the tumors caused by NF1 mutations are specific to nervous system-associated tissues, given that the pathways downstream of Ras-GTP (such as mTOR) are fairly ubiquitous/common across cell types. I would think that a loss of function mutation in NF1 producing a dominant inheritance pattern must mean that even partial loss of NF1 expression is extremely detrimental to these cells, but apparently not at all for other cell types. Why do you think this is? Is NF1 preferentially expressed in nervous system-associated tissues? I assume other cell types may express different, similar enzymes with GAP activity – but if so, why don’t mutations to these proteins produce similar conditions? (Or do they?) Let me know your thoughts!
admin · May 12, 2022 at 4:44 pm
Hello Miranda! Thank you for your comment, I hope you’ve recovered from Broad Street!
I concur with your concern! G-protein coupled receptors occupy the bulk of our genome, they are very important for cell signaling across all cell types. Loss of NF1 expression is detrimental to affected cells. Neurofibromin is exclusively produced in central nervous system (CNS) neurons.1 That is why neurofibromas grow along tumors, and most of the symptoms associated with NF1 are CNS-associated. Since a mutation in neurofibromin is what causes the disease, only CNS cells will experience the effects of this mutation. Diseases associated with other cell types that have mutations in enzymes with GAP activity are mostly cancer-causing, due to the enzyme’s role in controlling cellular proliferation. Here (https://www.cell.com/cell/pdf/S0092-8674(18)30298-8.pdf) is a link to a useful paper explaining the various mutations involving GTP hydrolysis that can cause cancer!
Bergoug M, Doudeau M, Godin F, Mosrin C, Vallée B, Bénédetti H. Neurofibromin Structure, Functions and Regulation. Cells. 2020;9(11):2365. Published 2020 Oct 27. doi:10.3390/cells9112365
Anne Bertolet · May 8, 2022 at 8:35 pm
Hello! You did a very nice job on a disease that can be quite variable. How does neurofibromatosis type 1 differ from type 2 in terms of mechanism?
Given the effect on GAP/Ras and cell signaling pathways, it would make sense on that this would lead to uncontrolled tumor growth. Is neurofibromin found in all cell types? Are neurofibromas the only tumor type that are found in this disease?
admin · May 12, 2022 at 4:47 pm
Thank you so much for your comment, Anne! The major difference between NF1 and NF2 is the mutated protein that causes the disease. In NF1, neurofibromin is mutated, restricting cells from regulating a proliferative pathway. In NF2, a protein called Merlin, is mutated. Merlin is a membrane-cytoskeleton scaffolding protein that is predominantly found in nervous tissue.1 It exhibits tumor-suppressive properties via contact inhibition.1 Similar to a neurofibromin deficiency, a deficiency in Merlin will cause affected cells to lose a system of proliferative regulation, increasing the chances for uncontrolled cell growth to occur.
Neurofibromin is exclusively produced in central nervous system (CNS) neurons.2 That is why neurofibromas grow along tumors, and most of the symptoms associated with NF1 are CNS-associated. Most of the tumors in NF1 are neurofibromas. However, these benign tumors have the potential to transform into malignant peripheral nerve sheet tumors (MPNSTs). Unlike neurofibromas, MPNSTs must be removed as soon as they are detected. The upregulation of epidermal growth factor receptor (EGFR) is linked to the progression of neurofibromas into MPNSTs.3
Pećina-Šlaus N. Merlin, the NF2 gene product. Pathol Oncol Res. 2013 Jul;19(3):365-73. doi: 10.1007/s12253-013-9644-y. Epub 2013 May 12. PMID: 23666797.
Bergoug M, Doudeau M, Godin F, Mosrin C, Vallée B, Bénédetti H. Neurofibromin Structure, Functions and Regulation. Cells. 2020;9(11):2365. Published 2020 Oct 27. doi:10.3390/cells9112365
Park, G.-H.; Lee, S.-J.; Lee, C.-G.; Kim, J.; Park, E.; Jeong, S.-Y. Neurofibromin Deficiency Causes Epidermal Growth Factor Receptor Upregulation through the Activation of Ras/ERK/SP1 Signaling Pathway in Neurofibromatosis Type 1-Associated Malignant Peripheral Nerve Sheet Tumor. Int. J. Mol. Sci. 2021, 22 (24), 13308. https://doi.org/10.3390/ijms222413308.
Tony Brach · May 11, 2022 at 12:56 am
Hi Mike,
Thanks for your research into neurofibromatosis! It was really interesting to me that pro-inflammatory pathways were up-regulated in these tumor cells, do you think that might have to do with increased need for cellular building blocks (ie hydrocarbons) used in cellular division? It was also interesting to see that a decrease in macrophages led to tumor regression, I would have thought the opposite? Do you have any insight into why this is? Thanks again for some really interesting research!