What is really supposed to happen? – The Healthy State
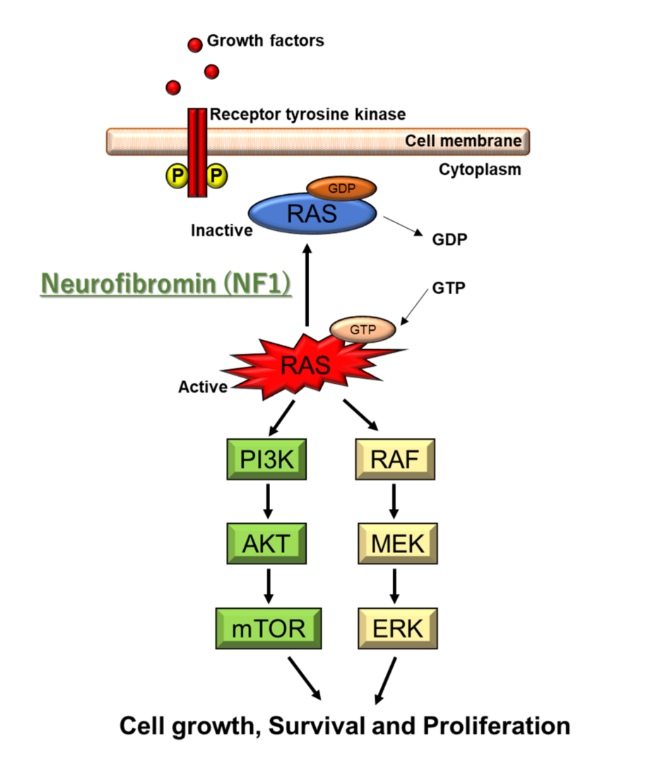
Cells have evolved a variety of ways to regulate themselves, recruit specific types of cells when needed and get rid of deleterious cells. These complex communication-based processes are conferred by cell-signaling pathways, mediated by many molecules in complex, interconnected ways. Biological homeostasis and survival is dependent on the ability of the body to ”check” itself. Disruptions to these processes may lead to the onset of disease, or even prove fatal depending on the severity of the disruption. Interrupting cell signaling pathways is a convenient way to cause a plethora of deleterious effects due to the interconnectedness of these pathways. The extensive effects of neurofibromatosis are due to the crucial role that functioning neurofibromin has in regulating a multitude of cellular pathways. The cellular pathways controlled by neurofibromin help regulate cell recruitment, proliferation, activation, and various signaling pathways related to the inflammatory and immune responses.
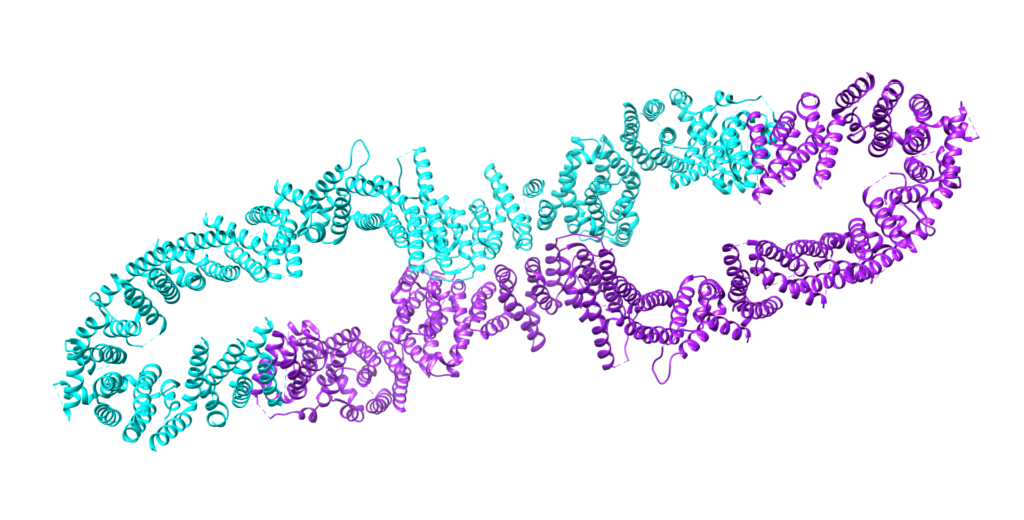
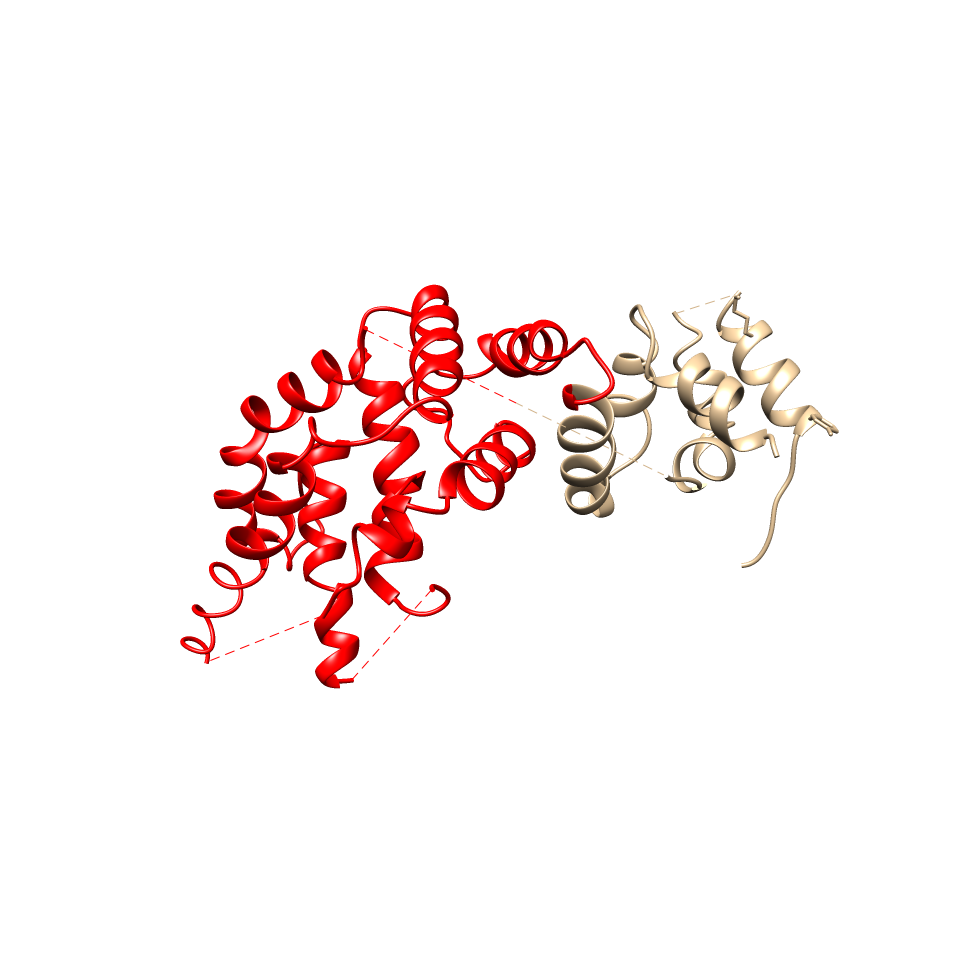
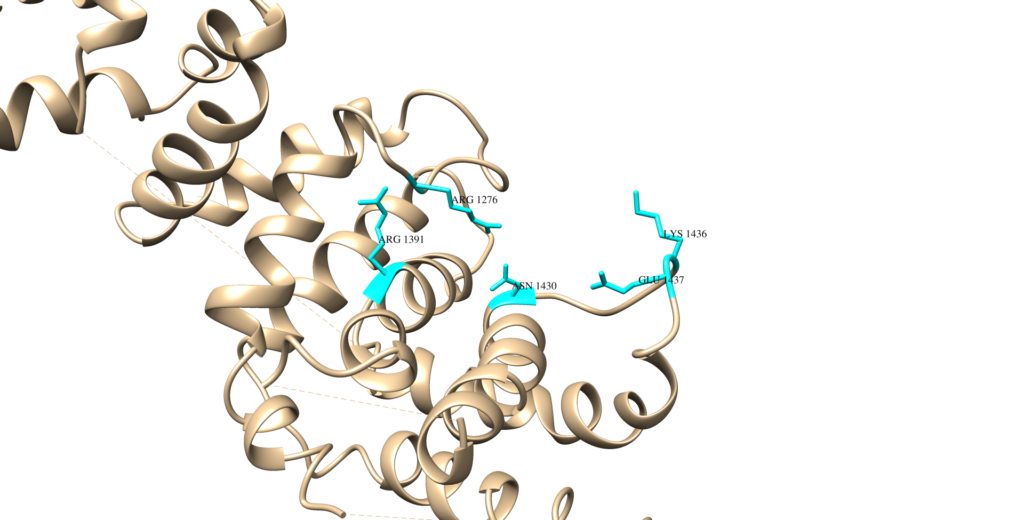
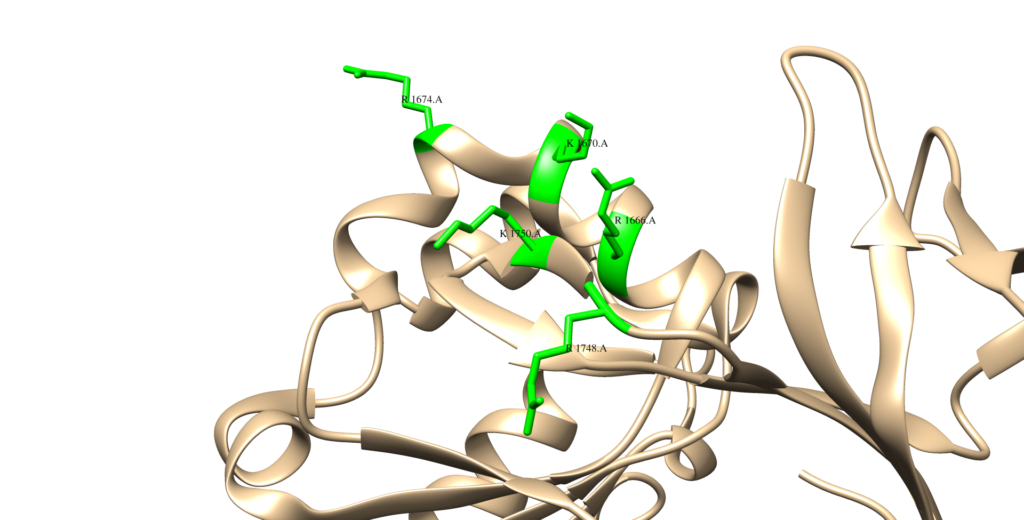
In its native state, neurofibromin is a 640-kDa protein that assembles into a homodimer.1 The two neurofibromin subunits that come together to form the homodimer are shown in teal and purple in the image above. As can be seen above, neurofibromin is comprised mostly of 𝝰-helices and loop regions connecting different areas of secondary structure, forming a figure eight-like structure. The neurofibromin protein contains many domains, including the N- and C-HEAT domains, the SEC-PH domain, and the GAP-related domain (GRD).1 The catalytic activity of neurofibromin can be attributed to the GRD. The precise way that Neurofibromin interacts with Ras is still poorly understood, and homologs to neurofibromin (other GTPases) are used to rationalize a structural relationship between Ras and neurofibromin. The minimum domain required for catalytic activity is believed to be a 230 residue fragment in the GRD (GAP-related domain), residues 1248 – 1477, shown in red below (this structure is just the GRD):2 The side chains of residues thought to interact with Ras are as follows (and shown in the Chimera image below as cyan residues): R1391, R1276, N1430, K1436, and E1437.2
As a GTPase, neurofibromin is crucial for regulating the activation of biomolecules in signaling pathways through phosphorylation. Neurofibromin helps regulate STAT3 signaling, the PLC pathway, and MAPK signaling pathways. These pathways are cytosolic, and are indispensable for proper functioning of cells. Therefore, each of them will be discussed individually:
MAPK Signaling Pathways: The mitogen-activated protein kinase (MAPK) family of signalling pathways, in simplest terms, is responsible for transmitting extracellular signals to intracellular targets.3 The MAPK singalling pathways are integral to the basic functioning of cells.3 In the case of neurofibromatosis, the RAS/ERK signalling pathway in MAPK is of most prevalence. Extracellular signal-regulated kinase (ERK) cascades play a significant role in cell differentiation and proliferation.3 In this signalling pathway, Ras (a G protein) is the upstream activating protein, which then activates Raf (a protein kinase), followed by MAPK.ERK kinase, finally followed by ERK.3 This pathway can be activated by Ca2+, receptor tyrosine kinase Ras, PKC, and G protein-coupled receptors.3 Once ERK is activated, it is translocated into the nucleus and controls the activity of various transcription factors.3 Neurofibromin inhibits Ras in the MAPL signaling pathway, preventing unregulated cell proliferation.
PLC Pathway: The phospholipase C (PLC) pathway is integral for the proper functioning of mast cells. The PLC pathway is triggered by a type III tyrosine kinase, KIT.4 KIT dimerizes, then is ligated to SCF (stem cell factor), which induces the PLC pathway via KIT phosphorylation of PLC.4 PLC is then responsible for the conversion of fatty acids of the cell membrane into signaling molecules. PLC converts phosphatidylinositol-4,5-bisphosphate (PIP2) into the cellular messengers inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG).5 IP3 then binds to receptors which cause the intracellular release of Ca2+, while DAG stays membrane-bound and activates protein kinase C upon an increase in intracellular Ca2+.5 Among their other functions, this pathway regulates cell proliferation and survival, key to immune signaling.5 Additionally, PLC activation is crucial to regulate ion channels.6 PLC mediates growth factor-induced cell signaling, resulting in cell proliferation. Furthermore, it is involved in cell migration and early secretory trafficking. It is overexpressed in cancer cells.7
STAT3 Signaling: Signal transducer and activator of transcription 3 (STAT3) plays a key role in development, tissue function, and mediating inflammation and immunity.8 STAT3 mediates important signal transduction cascades and has a myriad of biological roles.8 STAT3 has a myriad of roles in cellular processes such as the electron transport chain, for the purposes of this discussion, I will focus on the role that STAT3 plays in immunity. Upon pathogenic infection, STAT3 has a role in the recruitment of mature neutrophils to control the infection.8 STAT3 regulates key proliferative and neutrophil recruitment functions in the case of pathogenic infection.8 Furthermore, STAT3 also negatively inhibits this response, to prevent negative effects due to the inflammatory response.8 The activation and inhibition of STAT3 is controlled by a variety of cytokines such as IFN-gamma. STAT3 also plays a crucial role in the development of dendritic cells.8 STAT3 works to suppress signal transduction initiated by Toll-like receptors.8
The area of disease exertion (the nervous system) is indicative of the specific cells impacted by neurofibromatosis type 1. Defects in the NF1 gene have been found in neurofibromas, comprised of S100+ Schwann Cells, mast cells, and macrophages. Schwann cells are responsible for myelinating peripheral nerves and are the primary glial cells (non-neuronal cells in the nervous system).9 Numerous Schwann cells are required for myelination of the entire length of an axon.9 By myelinating axons, Schwann cells are able to increase the speed at which signals pass through the nervous system.9 Furthermore, they provide energy to axons and are crucial to their regeneration.9 Upon the death of axons, Schwann cells activate the expression of cytokines to recruit macrophages to the site of degeneration, and orchestrate the regeneration of the new axon.9 S100+ is a protein present in most Schwann cell tumors, and are believed to be integral to the pathogenesis of numerous cancers.10 There are many types of S100 proteins, Schwann cells contain S100B which is responsible for the stimulation of cellular proliferation, promoting inflammatory activities, and killing neurons through the use of reactive oxygen species.10 Mast cells are the largest population of inflammatory cells in cutaneous neurofibromas.11 They play a significant role in the immune response. Mast cells are able to recognize harmful antigens by binding to pathogens via Toll-like receptors on their surface.12 Upon recognition of pathogens, mast cells release a myriad of inflammatory mediators, targeting the pathogen that activated them.12 The mediators that are released by mast cells depend on the type of pathogen the mast cells recognizes (i.e. gram-negative bacteria vs. gram-positive bacteria).12 Mast cells are crucial for recruiting other immune cells such as “eosinophils, NK cells, neutrophils,” and T cells.12
Works Cited
To see all sources used, please access the Annotated Bibliography
0 Comments